
This might explain the occurrence of these conduction abnormalities in this patient at lower than usually expected as well.

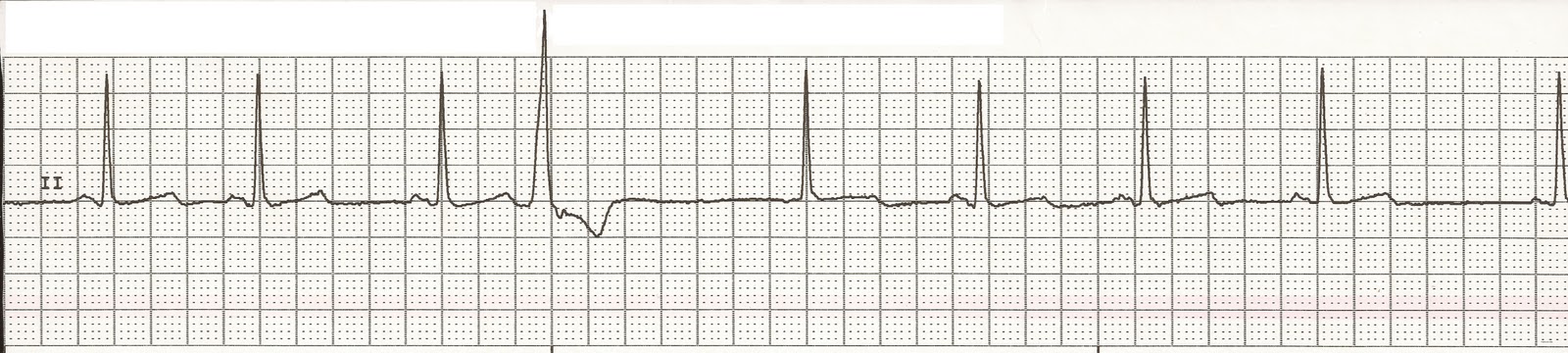
The "milder" (faster escape and quadrigeminy as opposed to bigeminy earlier) recurrence of this rhythm briefly with a normal potassium level suggests the possibility of intracellular or pericellular hyperkalemia affecting conduction despite "normal" potassium levels. Sick sinus syndrome is not excluded especially given his age and diabetes, but temporal correlation of resolution of the EKG abnormalities with management of hyperkalemia suggests hyperkalemia to be more likely. This is seen with digoxin, AV node blocking medications and sick sinus syndrome, but has not been reported due to hyperkalemia. Atrial bigeminy (and later quadrigeminy) in this patient is indicative of sinoatrial block allowing for a focus below the sinoatrial node to escape and allow for "escape capture bigeminy" seen in this patient. Junctional rhythm has been reported in the setting of hyperkalemia (6.5 mEq/L) with concurrent beta-blocker therapy, but our patient had neither been on AV node nor on beta-blocking agents. However, this patient’s serum sodium, calcium and magnesium levels were within normal limits. Hyponatremia and hypocalcemia have been shown to increase the sensitivity of the heart to the effects of hyperkalemia. This has been reported in milder hyperkalemia (5.5 mEq/L) as well, albeit latent sinus node dysfunction was not excluded in that patient. These occur at higher potassium levels (>8 mEq/L) because the sinoatrial and atrioventricular nodes are relatively resistant to hyperkalemia due to their sympathetic innervation. The second explanation is direct sinoventricular conduction through interatrial fibers bypassing atrial tissue due to non-excitability of atrial myocardium (sinoatrial block). The first possibility being severe sinus bradycardia (or "sinus arrest") due to a hypoexcitable pacemaker with junctional escape beat conducting to the ventricle. Ībsence of p waves in this patient has two possible explanations. As the RMP approaches threshold, the myocardium becomes hypoexcitable, which reduces sodium influx resulting in decrease of both the rate of rise and voltage of phase 0 of the action potential. These effects in turn reduce the speed of propagation of the action potential through the myocardium (wide QRS, prolonged PR interval) (Figure 4). This initially causes shortening of action potential duration (ST-T segment depression, peaked T waves and QT interval shortening). Hyperkalemia causes the RMP to become less negative due to decreased transmembrane concentration of potassium and has two prominent effects: it brings the RMP closer to threshold and increases potassium efflux (IKr) by increasing velocity of phase 3 repolarization. Resting membrane potential (RMP) mainly depends on the electrochemical potential generated by the concentration gradient of potassium across the cell membrane. This patient's electrocardiography (EKG) reveals transient severe sinus bradycardia with junctional escape at a lower potassium level than previously reported and capture bigeminy not reported with hyperkalemia. However, the patient continued to have an episodic junctional rhythm (Figure 3), but at a rate of 60 bpm, with atrial quadrigeminy coupled at 550 ms with normal T waves despite normal serum potassium (4.6 mEq/L) the next day and subsequently resolved over the next few days.
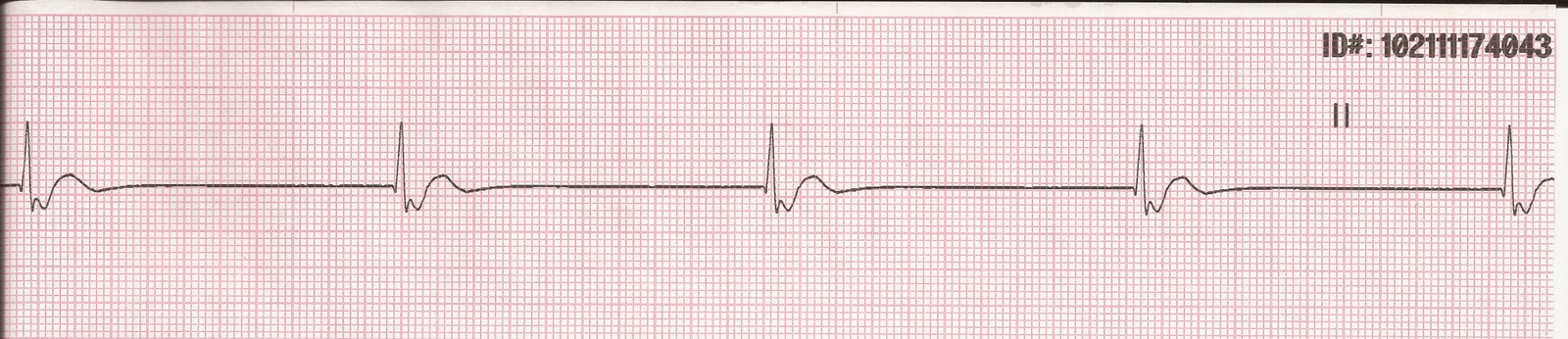
He was hemodynamically stable but admitted to the cardiac care unit where he was treated with hemodialysis resulting in restoration of normal sinus rhythm with resolution of APCs and normalization of T waves. The patient developed severe sinus bradycardia, with competing junctional rhythm at 52 bpm. Also noted were bigeminal atrial premature complexes (APCs) conducted with an incomplete left bundle branch block (QRS interval 110 ms), at regularly coupled intervals of 600 ms, along with peaked T waves (Figure 2). Other electrolytes (serum sodium (137-142 mEq/L), calcium (8-9 mg/dL) and magnesium (1.8-2.6 mg/dL)) remained within normal limits. After diuretics were initiated, his renal function worsened over 72 hours with an elevation in blood urea nitrogen/creatinine (from baseline of 39/2.8 to 96/4.3 mg/dL) and potassium increased from 4.7 to 5.8 mEq/L. The patient was initially in normal sinus rhythm (Figure 1). A 73-year-old gentleman with hypertension, type 2 diabetes mellitus, stage 4 chronic kidney disease, heart failure with left ventricular ejection fraction of 45% and hypothyroidism presented with progressive biventricular failure.


 0 kommentar(er)
0 kommentar(er)
